Regularised regression
Last updated on 2026-02-10 | Edit this page
Overview
Questions
- What is regularisation?
- How does regularisation work?
- How can we select the level of regularisation for a model?
Objectives
- Understand the benefits of regularised models.
- Understand how different types of regularisation work.
- Apply and critically analyse regularised regression models.
Introduction
This episode is about regularisation, also called regularised regression or penalised regression. This approach can be used for prediction and for feature selection and it is particularly useful when dealing with high-dimensional data.
One reason that we need special statistical tools for high-dimensional data is that standard linear models cannot handle high-dimensional data sets – one cannot fit a linear model where there are more features (predictor variables) than there are observations (data points). In the previous lesson, we dealt with this problem by fitting individual models for each feature and sharing information among these models. Now we will take a look at an alternative approach that can be used to fit models with more features than observations by stabilising coefficient estimates. This approach is called regularisation. Compared to many other methods, regularisation is also often very fast and can therefore be extremely useful in practice.
First, let us check out what happens if we try to fit a linear model
to high-dimensional data! We start by reading in the methylation
data from the last lesson:
R
library("SummarizedExperiment")
OUTPUT
Loading required package: MatrixGenericsOUTPUT
Loading required package: matrixStatsOUTPUT
Attaching package: 'MatrixGenerics'OUTPUT
The following objects are masked from 'package:matrixStats':
colAlls, colAnyNAs, colAnys, colAvgsPerRowSet, colCollapse,
colCounts, colCummaxs, colCummins, colCumprods, colCumsums,
colDiffs, colIQRDiffs, colIQRs, colLogSumExps, colMadDiffs,
colMads, colMaxs, colMeans2, colMedians, colMins, colOrderStats,
colProds, colQuantiles, colRanges, colRanks, colSdDiffs, colSds,
colSums2, colTabulates, colVarDiffs, colVars, colWeightedMads,
colWeightedMeans, colWeightedMedians, colWeightedSds,
colWeightedVars, rowAlls, rowAnyNAs, rowAnys, rowAvgsPerColSet,
rowCollapse, rowCounts, rowCummaxs, rowCummins, rowCumprods,
rowCumsums, rowDiffs, rowIQRDiffs, rowIQRs, rowLogSumExps,
rowMadDiffs, rowMads, rowMaxs, rowMeans2, rowMedians, rowMins,
rowOrderStats, rowProds, rowQuantiles, rowRanges, rowRanks,
rowSdDiffs, rowSds, rowSums2, rowTabulates, rowVarDiffs, rowVars,
rowWeightedMads, rowWeightedMeans, rowWeightedMedians,
rowWeightedSds, rowWeightedVarsOUTPUT
Loading required package: GenomicRangesOUTPUT
Loading required package: stats4OUTPUT
Loading required package: BiocGenericsOUTPUT
Attaching package: 'BiocGenerics'OUTPUT
The following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsOUTPUT
The following objects are masked from 'package:base':
anyDuplicated, aperm, append, as.data.frame, basename, cbind,
colnames, dirname, do.call, duplicated, eval, evalq, Filter, Find,
get, grep, grepl, intersect, is.unsorted, lapply, Map, mapply,
match, mget, order, paste, pmax, pmax.int, pmin, pmin.int,
Position, rank, rbind, Reduce, rownames, sapply, saveRDS, setdiff,
table, tapply, union, unique, unsplit, which.max, which.minOUTPUT
Loading required package: S4VectorsOUTPUT
Attaching package: 'S4Vectors'OUTPUT
The following object is masked from 'package:utils':
findMatchesOUTPUT
The following objects are masked from 'package:base':
expand.grid, I, unnameOUTPUT
Loading required package: IRangesOUTPUT
Loading required package: GenomeInfoDbOUTPUT
Loading required package: BiobaseOUTPUT
Welcome to Bioconductor
Vignettes contain introductory material; view with
'browseVignettes()'. To cite Bioconductor, see
'citation("Biobase")', and for packages 'citation("pkgname")'.OUTPUT
Attaching package: 'Biobase'OUTPUT
The following object is masked from 'package:MatrixGenerics':
rowMediansOUTPUT
The following objects are masked from 'package:matrixStats':
anyMissing, rowMediansWARNING
Warning: replacing previous import 'S4Arrays::makeNindexFromArrayViewport' by
'DelayedArray::makeNindexFromArrayViewport' when loading 'SummarizedExperiment'WARNING
Warning: multiple methods tables found for 'seqinfo'WARNING
Warning: multiple methods tables found for 'seqinfo<-'WARNING
Warning: multiple methods tables found for 'seqnames'R
methylation <- readRDS("data/methylation.rds")
## here, we transpose the matrix to have features as rows and samples as columns
methyl_mat <- t(assay(methylation))
age <- methylation$Age
Then, we try to fit a model with outcome age and all 5,000 features in this dataset as predictors (average methylation levels, M-values, across different sites in the genome).
R
# by using methyl_mat in the formula below, R will run a multivariate regression
# model in which each of the columns in methyl_mat is used as a predictor.
fit <- lm(age ~ methyl_mat)
summary(fit)
OUTPUT
Call:
lm(formula = age ~ methyl_mat)
Residuals:
ALL 37 residuals are 0: no residual degrees of freedom!
Coefficients: (4964 not defined because of singularities)
Estimate Std. Error t value Pr(>|t|)
(Intercept) 2640.474 NaN NaN NaN
methyl_matcg00075967 -108.216 NaN NaN NaN
methyl_matcg00374717 -139.637 NaN NaN NaN
methyl_matcg00864867 33.102 NaN NaN NaN
methyl_matcg00945507 72.250 NaN NaN NaN
[ reached 'max' / getOption("max.print") -- omitted 4996 rows ]
Residual standard error: NaN on 0 degrees of freedom
Multiple R-squared: 1, Adjusted R-squared: NaN
F-statistic: NaN on 36 and 0 DF, p-value: NAYou can see that we’re able to get some effect size estimates, but they seem very high! The summary also says that we were unable to estimate effect sizes for 4,964 features because of “singularities”. We clarify what singularities are in the note below but this means that R couldn’t find a way to perform the calculations necessary to fit the model. Large effect sizes and singularities are common when naively fitting linear regression models with a large number of features (i.e., to high-dimensional data), often since the model cannot distinguish between the effects of many, correlated features or when we have more features than observations.
Singularities
The message that lm() produced is not necessarily the
most intuitive. What are “singularities” and why are they an issue? A
singular matrix is one that cannot be inverted. R
uses inverse operations to fit linear models (find the coefficients)
using:
\[ (X^TX)^{-1}X^Ty, \]
where \(X\) is a matrix of predictor features and \(y\) is the outcome vector. Thus, if the matrix \(X^TX\) cannot be inverted to give \((X^TX)^{-1}\), R cannot fit the model and returns the error that there are singularities.
Why might R be unable to calculate \((X^TX)^{-1}\) and return the error that there are singularities? Well, when the determinant of the matrix is zero, we are unable to find its inverse. The determinant of the matrix is zero when there are more features than observations or often when the features are highly correlated.
R
xtx <- t(methyl_mat) %*% methyl_mat
det(xtx)
OUTPUT
[1] 0Challenge 1
Consider or discuss in groups:
- Why would we observe correlated features in high-dimensional biological data?
- Why might correlated features be a problem when fitting linear models?
- What issue might correlated features present when selecting features to include in a model one at a time?
- Many of the features in biological data represent very similar information biologically. For example, sets of genes that form complexes are often expressed in very similar quantities. Similarly, methylation levels at nearby sites are often very highly correlated.
- Correlated features can make inference unstable or even impossible mathematically.
- When we are selecting features one at a time we want to pick the most predictive feature each time. When a lot of features are very similar but encode slightly different information, which of the correlated features we select to include can have a huge impact on the later stages of model selection!
Regularisation can help us to deal with correlated features, as well as effectively reduce the number of features in our model. Before we describe regularisation, let’s recap what’s going on when we fit a linear model.
Coefficient estimates of a linear model
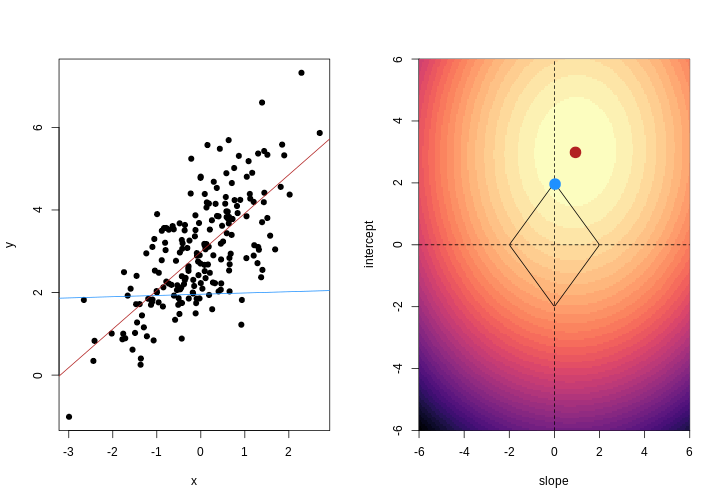
When we fit a linear model, we’re finding the line through our data that minimises the sum of the squared residuals. We can think of that as finding the slope and intercept that minimises the square of the length of the dashed lines. In this case, the red line in the left panel is the line that accomplishes this objective, and the red dot in the right panel is the point that represents this line in terms of its slope and intercept among many different possible models, where the background colour represents how well different combinations of slope and intercept accomplish this objective.
OUTPUT
Loading required package: viridisLite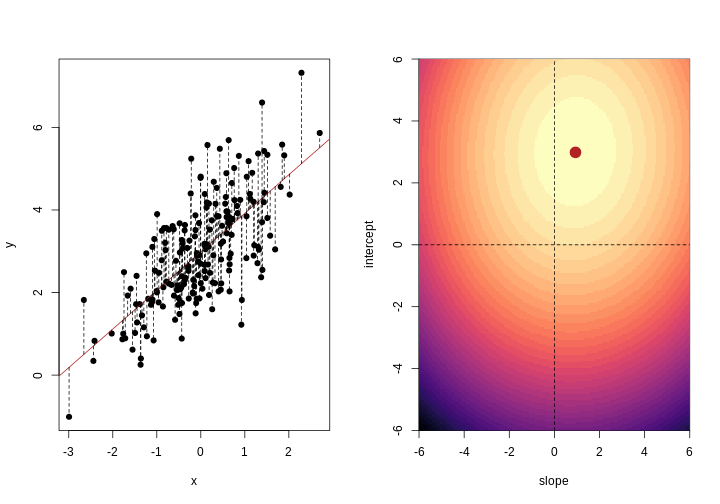
Mathematically, we can write the sum of squared residuals as
\[ \sum_{i=1}^N ( y_i-x'_i\beta)^2 \]
where \(\beta\) is a vector of (unknown) covariate effects which we want to learn by fitting a regression model: the \(j\)-th element of \(\beta\), which we denote as \(\beta_j\) quantifies the effect of the \(j\)-th covariate. For each individual \(i\), \(x_i\) is a vector of \(j\) covariate values and \(y_i\) is the true observed value for the outcome. The notation \(x'_i\beta\) indicates matrix multiplication. In this case, the result is equivalent to multiplying each element of \(x_i\) by its corresponding element in \(\beta\) and then calculating the sum across all of those values. The result of this product (often denoted by \(\hat{y}_i\)) is the predicted value of the outcome generated by the model. As such, \(y_i-x'_i\beta\) can be interpreted as the prediction error, also referred to as model residual. To quantify the total error across all individuals, we sum the square residuals \(( y_i-x'_i\beta)^2\) across all the individuals in our data.
Finding the value of \(\beta\) that minimises the sum above is the line of best fit through our data when considering this goal of minimising the sum of squared error. However, it is not the only possible line we could use! For example, we might want to err on the side of caution when estimating effect sizes (coefficients). That is, we might want to avoid estimating very large effect sizes.
Challenge 2
Discuss in groups:
- What are we minimising when we fit a linear model?
- Why are we minimising this objective? What assumptions are we making about our data when we do so?
- When we fit a linear model we are minimising the squared error. In fact, the standard linear model estimator is often known as “ordinary least squares”. The “ordinary” really means “original” here, to distinguish between this method, which dates back to ~1800, and some more “recent” (think 1940s…) methods.
- Squared error is useful because it ignores the sign of the residuals (whether they are positive or negative). It also penalises large errors much more than small errors. On top of all this, it also makes the solution very easy to compute mathematically. Least squares assumes that, when we account for the change in the mean of the outcome based on changes in the income, the data are normally distributed. That is, the residuals of the model, or the error left over after we account for any linear relationships in the data, are normally distributed, and have a fixed variance.
Model selection using training and test sets
Sets of models are often compared using statistics such as adjusted \(R^2\), AIC or BIC. These show us how well the model is learning the data used in fitting that same model 1. However, these statistics do not really tell us how well the model will generalise to new data. This is an important thing to consider – if our model doesn’t generalise to new data, then there’s a chance that it’s just picking up on a technical or batch effect in our data, or simply some noise that happens to fit the outcome we’re modelling. This is especially important when our goal is prediction – it’s not much good if we can only predict well for samples where the outcome is already known, after all!
To get an idea of how well our model generalises, we can split the data into two - a “training” and a “test” set. We use the “training” data to fit the model, and then see its performance on the “test” data.
One thing that often happens in this context is that large coefficient values minimise the training error, but they don’t minimise the test error on unseen data. First, we’ll go through an example of what exactly this means.
To compare the training and test errors for a model of methylation features and age, we’ll split the data into training and test sets, fit a linear model and calculate the errors. First, let’s calculate the training error. Let’s start by splitting the data into training and test sets:
R
methylation <- readRDS("/data/methylation.rds")
WARNING
Warning in gzfile(file, "rb"): cannot open compressed file
'/data/methylation.rds', probable reason 'No such file or directory'ERROR
Error in gzfile(file, "rb"): cannot open the connectionR
library("SummarizedExperiment")
age <- methylation$Age
methyl_mat <- t(assay(methylation))
We will then subset the data to a set of CpG markers that are known to be associated with age from a previous study by Horvath et al.2, described in data.
R
cpg_markers <- c("cg16241714", "cg14424579", "cg22736354", "cg02479575", "cg00864867",
"cg25505610", "cg06493994", "cg04528819", "cg26297688", "cg20692569",
"cg04084157", "cg22920873", "cg10281002", "cg21378206", "cg26005082",
"cg12946225", "cg25771195", "cg26845300", "cg06144905", "cg27377450"
)
horvath_mat <- methyl_mat[, cpg_markers]
## Generate an index to split the data
set.seed(42)
train_ind <- sample(nrow(methyl_mat), 25)
## Split the data
train_mat <- horvath_mat[train_ind, ]
train_age <- age[train_ind]
test_mat <- horvath_mat[-train_ind, ]
test_age <- age[-train_ind]
Now let’s fit a linear model to our training data and calculate the training error. Here we use the mean of the squared difference between our predictions and the observed data, or “mean squared error” (MSE).
R
## Fit a linear model
# as.data.frame() converts train_mat into a data.frame
# Using the `.` syntax above together with a `data` argument will lead to
# the same result as using `train_age ~ train_mat`: R will fit a multivariate
# regression model in which each of the columns in `train_mat` is used as
# a predictor. We opted to use the `.` syntax because it will help us to
# obtain model predictions using the `predict()` function.
fit_horvath <- lm(train_age ~ ., data = as.data.frame(train_mat))
## Function to calculate the (mean squared) error
mse <- function(true, prediction) {
mean((true - prediction)^2)
}
## Calculate the training error
err_lm_train <- mse(train_age, fitted(fit_horvath))
err_lm_train
OUTPUT
[1] 1.319628The training error appears very low here – on average we’re only off by about a year!
Challenge 3
For the fitted model above, calculate the mean squared error for the test set.
First, let’s find the predicted values for the ‘unseen’ test data:
R
pred_lm <- predict(fit_horvath, newdata = as.data.frame(test_mat))
The mean squared error for the test set is the mean of the squared error between the predicted values and true test data.
R
err_lm <- mse(test_age, pred_lm)
err_lm
OUTPUT
[1] 223.3571Unfortunately, the test error is a lot higher than the training error. If we plot true age against predicted age for the samples in the test set, we can gain more insight into the performance of the model on the test set. Ideally, the predicted values should be close to the test data.
R
par(mfrow = c(1, 1))
plot(test_age, pred_lm, pch = 19)
abline(coef = 0:1, lty = "dashed")
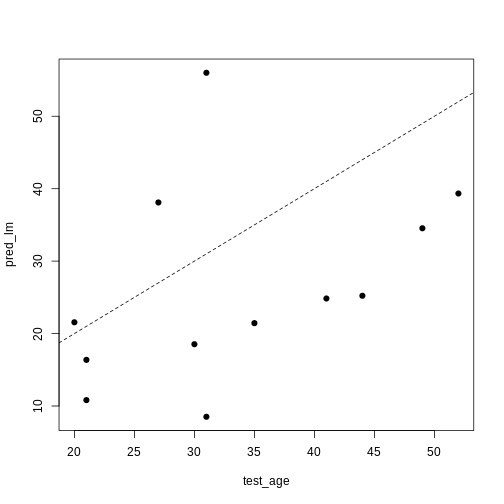
This figure shows the predicted ages obtained from a linear model fit plotted against the true ages, which we kept in the test dataset. If the prediction were good, the dots should follow a line. Regularisation can help us to make the model more generalisable, improving predictions for the test dataset (or any other dataset that is not used when fitting our model).
Regularisation
Regularisation can be used to reduce correlation between predictors, the number of features, and improve generalisability by restricting model parameter estimates. Essentially, we add another condition to the problem we’re solving with linear regression that controls the total size of the coefficients that come out and shrinks many coefficients to zero (or near zero).
For example, we might say that the point representing the slope and intercept must fall within a certain distance of the origin, \((0, 0)\). For the 2-parameter model (slope and intercept), we could visualise this constraint as a circle with a given radius. We want to find the “best” solution (in terms of minimising the residuals) that also falls within a circle of a given radius (in this case, 2).
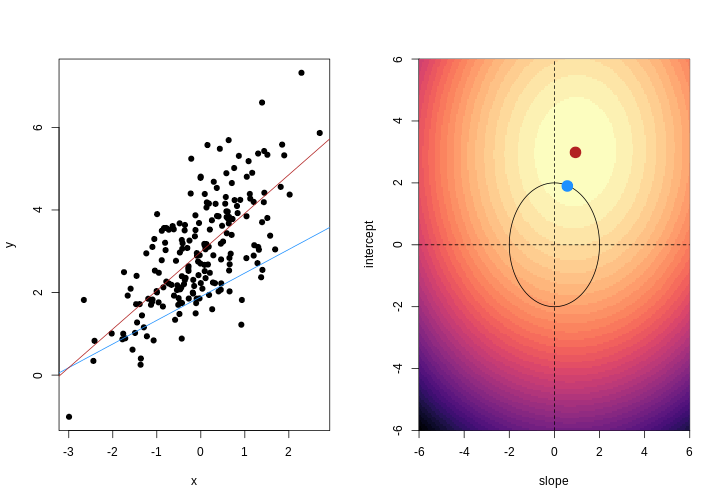
Ridge regression
There are multiple ways to define the distance that our solution must fall in. The one we’ve plotted above controls the squared sum of the coefficients, \(\beta\). This is also sometimes called the \(L^2\) norm. This is defined as
\[ \left\lVert \beta\right\lVert_2 = \sqrt{\sum_{j=1}^p \beta_j^2} \]
To control this, we specify that the solution for the equation above also has to have an \(L^2\) norm smaller than a certain amount. Or, equivalently, we try to minimise a function that includes our \(L^2\) norm scaled by a factor that is usually written \(\lambda\).
\[ \sum_{i=1}^N \biggl( y_i - x'_i\beta\biggr)^2 + \lambda \left\lVert \beta \right\lVert_2 ^2 \]
This type of regularisation is called ridge regression. Another way of thinking about this is that when finding the best model, we’re weighing up a balance of the ordinary least squares objective and a “penalty” term that punishes models with large coefficients. The balance between the penalty and the ordinary least squares objective is controlled by \(\lambda\) - when \(\lambda\) is large, we want to penalise large coefficients. In other words, we care a lot about the size of the coefficients and we want to reduce the complexity of our model. When \(\lambda\) is small, we don’t really care a lot about shrinking our coefficients and we opt for a more complex model. When it’s zero, we’re back to just using ordinary least squares. We see how a penalty term, \(\lambda\), might be chosen later in this episode.
For now, to see how regularisation might improve a model, let’s fit a
model using the same set of 20 features (stored as
cpg_markers) selected earlier in this episode (these are a
subset of the features identified by Horvarth et al), using both
regularised and ordinary least squares. To fit regularised regression
models, we will use the glmnet
package.
R
library("glmnet")
OUTPUT
Loading required package: MatrixOUTPUT
Attaching package: 'Matrix'OUTPUT
The following object is masked from 'package:S4Vectors':
expandOUTPUT
Loaded glmnet 4.1-10R
## glmnet() performs scaling by default, supply un-scaled data:
horvath_mat <- methyl_mat[, cpg_markers] # select the same 20 sites as before
train_mat <- horvath_mat[train_ind, ] # use the same individuals as selected before
test_mat <- horvath_mat[-train_ind, ]
ridge_fit <- glmnet(x = train_mat, y = train_age, alpha = 0)
plot(ridge_fit, xvar = "lambda")
abline(h = 0, lty = "dashed")
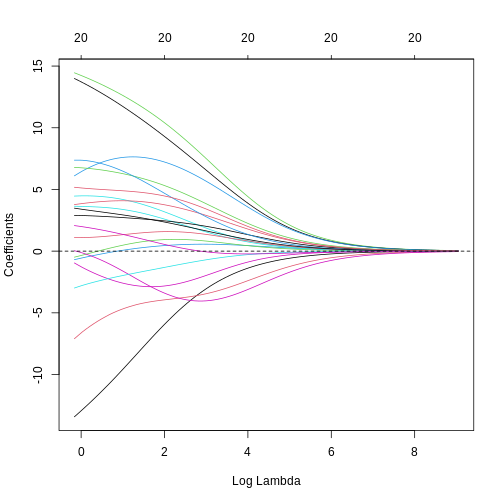
This plot shows how the estimated coefficients for each CpG site change as we increase the penalty, \(\lambda\). That is, as we decrease the size of the region that solutions can fall into, the values of the coefficients that we get back tend to decrease. In this case, coefficients tend towards zero but generally don’t reach it until the penalty gets very large. We can see that initially, some parameter estimates are really, really large, and these tend to shrink fairly rapidly.
We can also notice that some parameters “flip signs”; that is, they start off positive and become negative as lambda grows. This is a sign of collinearity, or correlated predictors. As we reduce the importance of one feature, we can “make up for” the loss in accuracy from that one feature by adding a bit of weight to another feature that represents similar information.
Since we split the data into test and training data, we can prove that ridge regression predicts the test data better than the model with no regularisation. Let’s generate our predictions under the ridge regression model and calculate the mean squared error in the test set:
R
# Obtain a matrix of predictions from the ridge model,
# where each column corresponds to a different lambda value
pred_ridge <- predict(ridge_fit, newx = test_mat)
# Calculate MSE for every column of the prediction matrix against the vector of true ages
err_ridge <- apply(pred_ridge, 2, function(col) mse(test_age, col))
min_err_ridge <- min(err_ridge)
# Identify the lambda value that results in the lowest MSE (ie, the "best" lambda value)
which_min_err <- which.min(err_ridge)
pred_min_ridge <- pred_ridge[, which_min_err]
## Return errors
min_err_ridge
OUTPUT
[1] 46.76802This is much lower than the test error for the model without regularisation:
R
err_lm
OUTPUT
[1] 223.3571We can see where on the continuum of lambdas we’ve picked a model by plotting the coefficient paths again. In this case, we’ve picked a model with fairly modest coefficient shrinking.
R
chosen_lambda <- ridge_fit$lambda[which.min(err_ridge)]
plot(ridge_fit, xvar = "lambda")
abline(v = log(chosen_lambda), lty = "dashed")
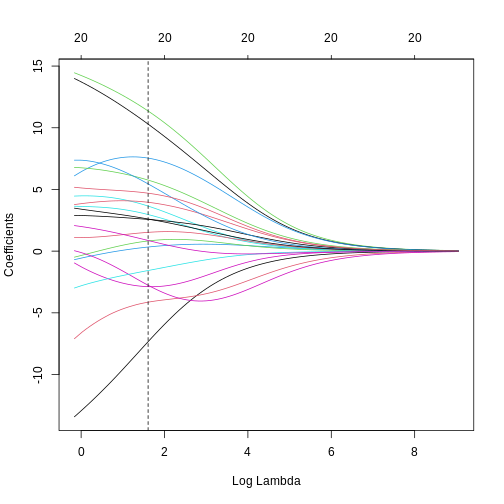
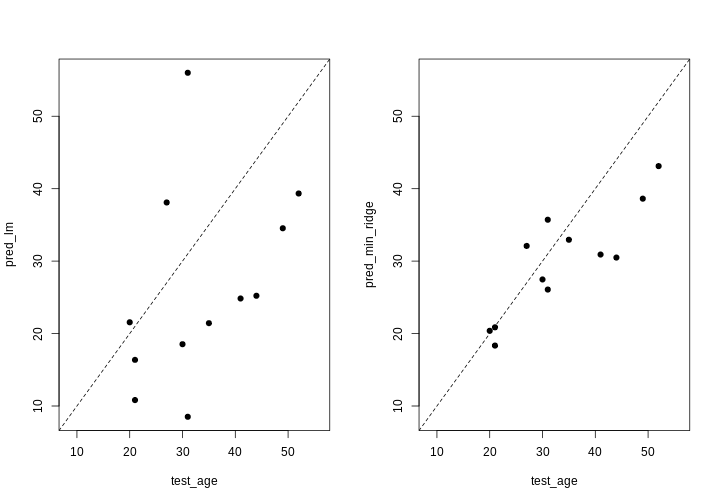
Challenge 4
- Which performs better, ridge or OLS?
- Plot predicted ages for each method against the true ages. How do the predictions look for both methods? Why might ridge be performing better?
- Ridge regression performs significantly better on unseen data, despite being “worse” on the training data.
R
min_err_ridge
OUTPUT
[1] 46.76802R
err_lm
OUTPUT
[1] 223.3571- The ridge ones are much less spread out with far fewer extreme predictions.
R
all <- c(pred_lm, test_age, pred_min_ridge)
lims <- range(all)
par(mfrow = 1:2)
plot(test_age, pred_lm,
xlim = lims, ylim = lims,
pch = 19
)
abline(coef = 0:1, lty = "dashed")
plot(test_age, pred_min_ridge,
xlim = lims, ylim = lims,
pch = 19
)
abline(coef = 0:1, lty = "dashed")
LASSO regression
LASSO is another type of regularisation. In this case we use the \(L^1\) norm, or the sum of the absolute values of the coefficients.
\[ \left\lVert \beta \right\lVert_1 = \sum_{j=1}^p |\beta_j| \]
This tends to produce sparse models; that is to say, it tends to remove features from the model that aren’t necessary to produce accurate predictions. This is because the region we’re restricting the coefficients to has sharp edges. So, when we increase the penalty (reduce the norm), it’s more likely that the best solution that falls in this region will be at the corner of this diagonal (i.e., one or more coefficient is exactly zero).
Challenge 5
- Use
glmnet()to fit a LASSO model (hint: setalpha = 1). - Plot the model object. Remember that for ridge regression, we set
xvar = "lambda". What if you don’t set this? What’s the relationship between the two plots? - How do the coefficient paths differ to the ridge case?
- Fitting a LASSO model is very similar to a ridge model, we just need
to change the
alphasetting.
R
fit_lasso <- glmnet(x = methyl_mat, y = age, alpha = 1)
- When
xvar = "lambda", the x-axis represents increasing model sparsity from left to right, because increasing lambda increases the penalty added to the coefficients. When we instead plot the L1 norm on the x-axis, increasing L1 norm means that we are allowing our coefficients to take on increasingly large values.
R
par(mfrow = c(1, 2))
plot(fit_lasso, xvar = "lambda")
plot(fit_lasso)
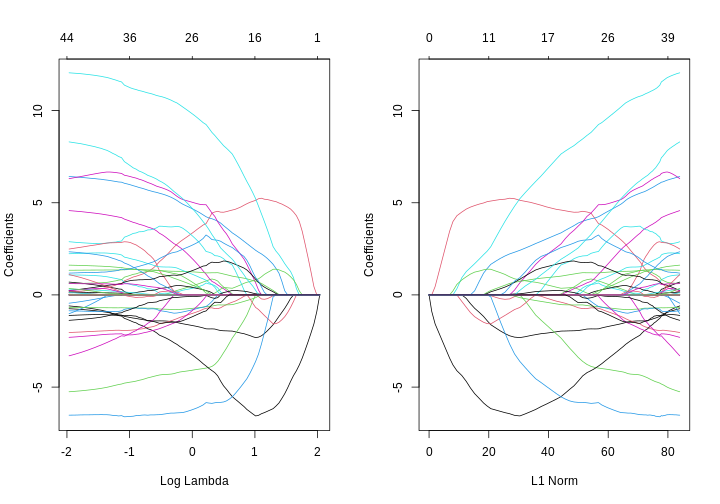
- When using LASSO, the paths tend to go to exactly zero much more as the penalty ($ \lambda $) increases. In the ridge case, the paths tend towards zero but less commonly reach exactly zero.
Cross-validation to find the best value of \(\lambda\)
Ideally, we want \(\lambda\) to be large enough to reduce the complexity of our model, thus reducing the number of and correlations between the features, and improving generalisability. However, we don’t want the value of \(\lambda\) to be so large that we lose a lot of the valuable information in the features.
Various methods can be used to balance this trade-off and thus select the “best” value for \(\lambda\). One method splits the data into \(K\) chunks. We then use \(K-1\) of these as the training set, and the remaining \(1\) chunk as the test set. We can repeat this until we’ve rotated through all \(K\) chunks, giving us a good estimate of how well each of the lambda values work in our data. This is called cross-validation, and doing this repeated test/train split gives us a better estimate of how generalisable our model is.
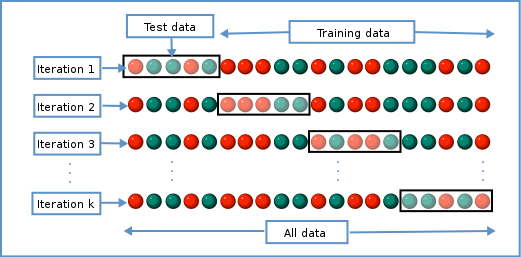
We can use this new idea to choose a lambda value by finding the lambda that minimises the error across each of the test and training splits. In R:
R
# fit lasso model with cross-validation across a range of lambda values
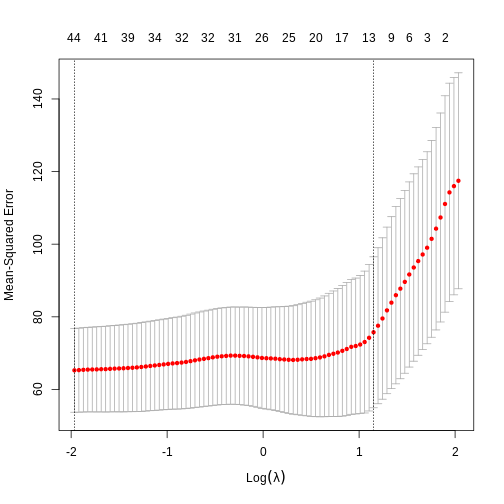
lasso <- cv.glmnet(methyl_mat, age, alpha = 1)
plot(lasso)
R
# Extract the coefficients from the model with the lowest mean squared error from cross-validation
coefl <- coef(lasso, lasso$lambda.min)
# select only non-zero coefficients
selection <- which(coefl != 0)
# and convert to a normal matrix
selected_coefs <- as.matrix(coefl)[selection, 1]
selected_coefs
OUTPUT
(Intercept) cg02388150 cg06493994 cg22449114 cg22736354
-8.4133296328 0.6966503392 0.1615535465 6.4255580409 12.0507794749
cg03330058 cg09809672 cg11299964 cg19761273 cg26162695
-0.0002362055 -0.7487594618 -2.0399663416 -5.2538055304 -0.4486970332
cg09502015 cg24771684 cg08446924 cg13215762 cg24549277
1.0787003366 4.5743800395 -0.5960137381 0.1481402638 0.6290915767
cg12304482 cg13131095 cg17962089 cg13842639 cg04080666
-1.0167896196 2.8860222552 6.3065284096 0.1590465147 2.4889065761
cg06147194 cg03669936 cg14230040 cg19848924 cg23964682
-0.6838637838 -0.0352696698 0.1280760909 -0.0006938337 1.3378854603
cg13578928 cg02745847 cg17410295 cg17459023 cg06223736
-0.8601170264 2.2346315955 -2.3008028295 0.0370389967 1.6158734083
cg06717750 cg20138604 cg12851161 cg20972027 cg23878422
2.3401693309 0.0084327521 -3.3033355652 0.2442751682 1.1059030593
cg16612298 cg03762081 cg14428146 cg16908369 cg16271524
0.0050053190 -6.5228858163 0.3167227488 0.2302773154 -1.3787104336
cg22071651 cg04262805 cg24969251 cg11233105 cg03156032
0.3480551279 1.1841804186 8.3024629942 0.6130598151 -1.1121959544 We can see that cross-validation has selected a value of \(\lambda\) resulting in 44 features and the intercept.
Elastic nets: blending ridge regression and the LASSO
So far, we’ve used ridge regression (where alpha = 0)
and LASSO regression, (where alpha = 1). What if
alpha is set to a value between zero and one? Well, this
actually lets us blend the properties of ridge and LASSO regression.
This allows us to have the nice properties of the LASSO, where
uninformative variables are dropped automatically, and the nice
properties of ridge regression, where our coefficient estimates are a
bit more conservative, and as a result our predictions are a bit
better.
Formally, the objective function of elastic net regression is to optimise the following function:
\[ \left(\sum_{i=1}^N y_i - x'_i\beta\right) + \lambda \left( \alpha \left\lVert \beta \right\lVert_1 + (1-\alpha) \left\lVert \beta \right\lVert_2 \right) \]
You can see that if alpha = 1, then the L1 norm term is
multiplied by one, and the L2 norm is multiplied by zero. This means we
have pure LASSO regression. Conversely, if alpha = 0, the
L2 norm term is multiplied by one, and the L1 norm is multiplied by
zero, meaning we have pure ridge regression. Anything in between gives
us something in between.
The contour plots we looked at previously to visualise what this
penalty looks like for different values of alpha.
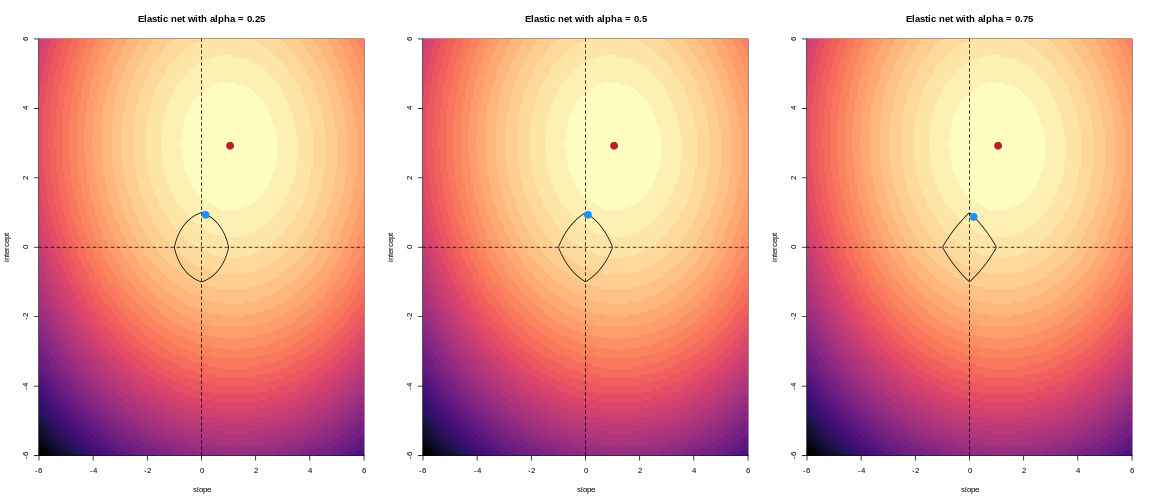
Challenge 6
- Fit an elastic net model (hint: alpha = 0.5) without cross-validation and plot the model object.
- Fit an elastic net model with cross-validation and plot the error. Compare with LASSO.
- Select the lambda within one standard error of the minimum
cross-validation error (hint:
lambda.1se). Compare the coefficients with the LASSO model. - Discuss: how could we pick an
alphain the range (0, 1)? Could we justify choosing one a priori?
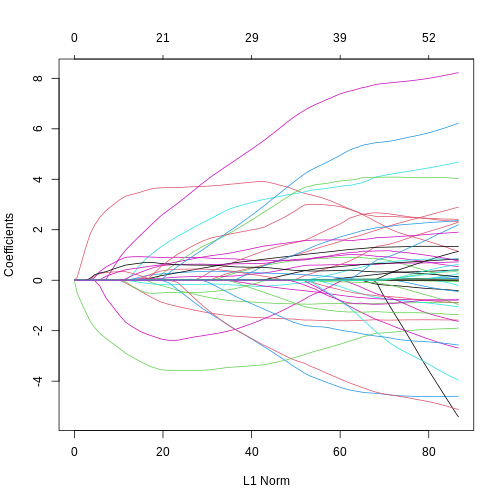
- Fitting an elastic net model is just like fitting a LASSO model. You can see that coefficients tend to go exactly to zero, but the paths are a bit less extreme than with pure LASSO; similar to ridge.
R
elastic <- glmnet(methyl_mat[, -1], age, alpha = 0.5)
plot(elastic)
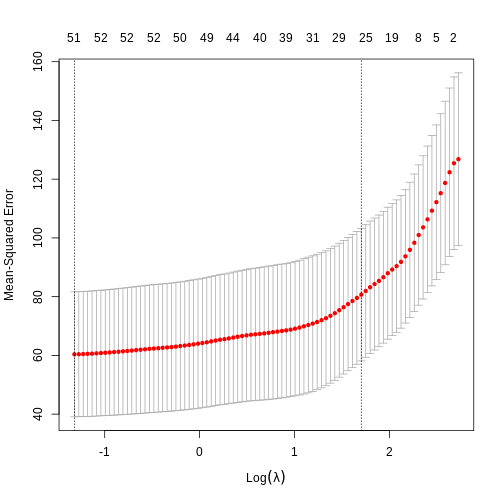
- The process of model selection is similar for elastic net models as for LASSO models.
R
elastic_cv <- cv.glmnet(methyl_mat[, -1], age, alpha = 0.5)
plot(elastic_cv)
- You can see that the coefficients from these two methods are broadly similar, but the elastic net coefficients are a bit more conservative. Further, more coefficients are exactly zero in the LASSO model.
R
coefe <- coef(elastic_cv, elastic_cv$lambda.1se)
sum(coefe[, 1] == 0)
OUTPUT
[1] 4973R
sum(coefl[, 1] == 0)
OUTPUT
[1] 4956R
plot(
coefl[, 1], coefe[, 1],
pch = 16,
xlab = "LASSO coefficients",
ylab = "Elastic net coefficients"
)
ERROR
Error in xy.coords(x, y, xlabel, ylabel, log): 'x' and 'y' lengths differR
abline(0:1, lty = "dashed")
ERROR
Error in int_abline(a = a, b = b, h = h, v = v, untf = untf, ...): plot.new has not been called yet- You could pick an arbitrary value of
alpha, because arguably pure ridge regression or pure LASSO regression are also arbitrary model choices. To be rigorous and to get the best-performing model and the best inference about predictors, it’s usually best to find the best combination ofalphaandlambdausing a grid search approach in cross-validation. However, this can be very computationally demanding.
The bias-variance tradeoff
When we make predictions in statistics, there are two sources of error that primarily influence the (in)accuracy of our predictions. these are bias and variance.
The total expected error in our predictions is given by the following equation:
\[ E(y - \hat{y}) = \text{Bias}^2 + \text{Variance} + \sigma^2 \]
Here, \(\sigma^2\) represents the irreducible error, that we can never overcome. Bias results from erroneous assumptions in the model used for predictions. Fundamentally, bias means that our model is mis-specified in some way, and fails to capture some components of the data-generating process (which is true of all models). If we have failed to account for a confounding factor that leads to very inaccurate predictions in a subgroup of our population, then our model has high bias.
Variance results from sensitivity to particular properties of the input data. For example, if a tiny change to the input data would result in a huge change to our predictions, then our model has high variance.
Linear regression is an unbiased model under certain conditions. In fact, the Gauss-Markov theorem shows that under the right conditions, OLS is the best possible type of unbiased linear model.
Introducing penalties to means that our model is no longer unbiased, meaning that the coefficients estimated from our data will systematically deviate from the ground truth. Why would we do this? As we saw, the total error is a function of bias and variance. By accepting a small amount of bias, it’s possible to achieve huge reductions in the total expected error.
This bias-variance tradeoff is also why people often favour elastic net regression over pure LASSO regression.
Other types of outcomes
You may have noticed that glmnet is written as
glm, not lm. This means we can actually model
a variety of different outcomes using this regularisation approach. For
example, we can model binary variables using logistic regression, as
shown below. The type of outcome can be specified using the
family argument, which specifies the family of the outcome
variable.
In fact, glmnet is somewhat cheeky as it also allows you
to model survival using Cox proportional hazards models, which aren’t
GLMs, strictly speaking.
For example, in the current dataset we can model smoking status as a
binary variable in logistic regression by setting
family = "binomial".
The package documentation explains this in more detail.
R
smoking <- as.numeric(factor(methylation$smoker)) - 1
# binary outcome
table(smoking)
OUTPUT
smoking
0 1
30 7 R
fit <- cv.glmnet(x = methyl_mat, nfolds = 5, y = smoking, family = "binomial")
WARNING
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous ground
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous ground
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous ground
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous ground
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous ground
Warning in lognet(x, is.sparse, y, weights, offset, alpha, nobs, nvars, : one
multinomial or binomial class has fewer than 8 observations; dangerous groundR
coef <- coef(fit, s = fit$lambda.min)
coef <- as.matrix(coef)
coef[which(coef != 0), 1]
OUTPUT
[1] -1.455287R
plot(fit)
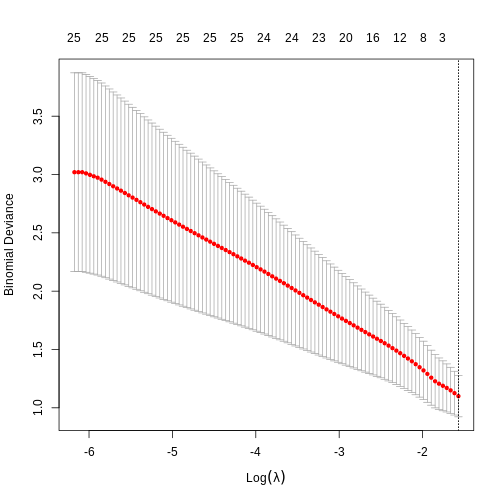
In this case, the results aren’t very interesting! We select an intercept-only model. However, as highlighted by the warnings above, we should not trust this result too much as the data was too small to obtain reliable results! We only included it here to provide the code that could be used to perform penalised regression for binary outcomes (i.e. penalised logistic regression).
tidymodels
A lot of the packages for fitting predictive models like regularised regression have different user interfaces. To do predictive modelling, it’s important to consider things like choosing a good performance metric and how to run normalisation. It’s also useful to compare different model “engines”.
To this end, the tidymodels R framework
exists. We’re not doing a course on advanced topics in predictive
modelling so we are not covering this framework in detail. However, the
code below would be useful to perform repeated cross-validation. More
information about tidymodels, including
installation instructions, can be found on the tidymodels website.
R
library("tidymodels")
all_data <- as.data.frame(cbind(age = age, methyl_mat))
split_data <- initial_split(all_data)
norm_recipe <- recipe(training(split_data)) %>%
## everything other than age is a predictor
update_role(everything(), new_role = "predictor") %>%
update_role(age, new_role = "outcome") %>%
## center and scale all the predictors
step_center(all_predictors()) %>%
step_scale(all_predictors())
## set the "engine" to be a linear model with tunable alpha and lambda
glmnet_model <- linear_reg(penalty = tune(), mixture = tune()) %>%
set_engine("glmnet")
## define a workflow, with normalisation recipe into glmnet engine
workflow <- workflow() %>%
add_recipe(norm_recipe) %>%
add_model(glmnet_model)
## 5-fold cross-validation repeated 5 times
folds <- vfold_cv(training(split_data), v = 5, repeats = 5)
## define a grid of lambda and alpha parameters to search
glmn_set <- parameters(
penalty(range = c(-5, 1), trans = log10_trans()),
mixture()
)
glmn_grid <- grid_regular(glmn_set)
ctrl <- control_grid(save_pred = TRUE, verbose = TRUE)
## use the metric "rmse" (root mean squared error) to grid search for the
## best model
results <- workflow %>%
tune_grid(
resamples = folds,
metrics = metric_set(rmse),
control = ctrl
)
## select the best model based on RMSE
best_mod <- results %>% select_best("rmse")
best_mod
## finalise the workflow and fit it with all of the training data
final_workflow <- finalize_workflow(workflow, best_mod)
final_workflow
final_model <- final_workflow %>%
fit(data = training(split_data))
## plot predicted age against true age for test data
plot(
testing(split_data)$age,
predict(final_model, new_data = testing(split_data))$.pred,
xlab = "True age",
ylab = "Predicted age",
pch = 16,
log = "xy"
)
abline(0:1, lty = "dashed")
Further reading
Footnotes
- Regularisation is a way to fit a model, get better estimates of effect sizes, and perform variable selection simultaneously.
- Test and training splits, or cross-validation, are a useful way to select models or hyperparameters.
- Regularisation can give us a more predictive set of variables, and by restricting the magnitude of coefficients, can give us a better (and more stable) estimate of our outcome.
- Regularisation is often very fast! Compared to other methods for variable selection, it is very efficient. This makes it easier to practice rigorous variable selection.
Model selection including \(R^2\), AIC and BIC are covered in the additional feature selection for regression episode of this course.↩︎